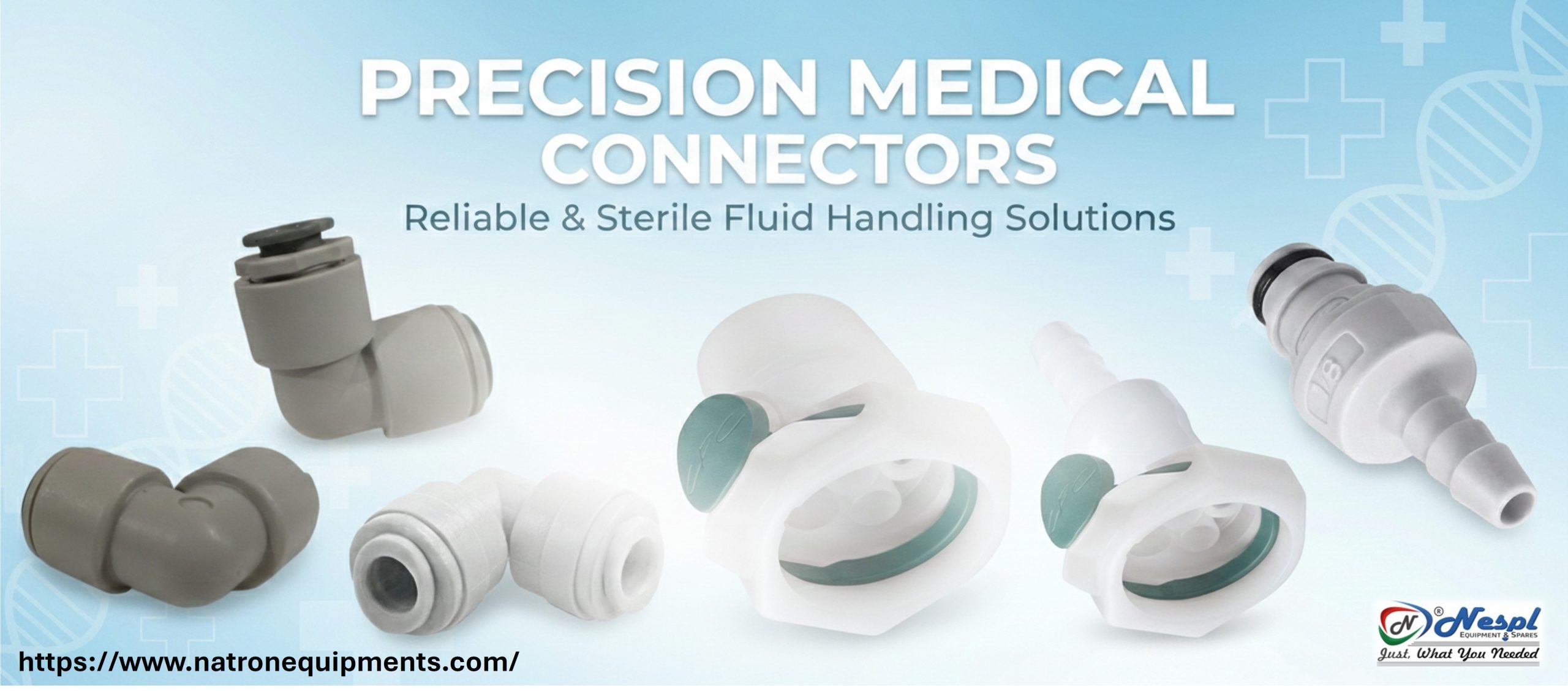
In biopharma manufacturing, reliability is not optional. Every component — from major equipment assemblies to small replacement parts — directly impacts product quality, regulatory compliance, and operational continuity.
Selecting the right supplier is not just a purchasing decision. It is a strategic decision that affects validation, uptime, and long-term process stability.
For any doubt & Query write us at: sales3@natronequipments.com
NESPL supports biopharma facilities by supplying specialized equipment and precision spare parts designed for regulated manufacturing environments. Here’s what sets NESPL apart.
Focused on Biopharma Requirements
Biopharma environments demand more than general industrial components. Sterility, traceability, validation compatibility, and process repeatability are critical.
NESPL supplies equipment and spare parts aligned with:
- GMP-regulated production standards
- Cleanroom and sterile process environments
- Biopharma-grade material requirements
- Equipment compatibility for regulated facilities
This focused approach ensures that the components supplied are suitable for controlled manufacturing settings.
Reliable Equipment and Precision Spare Parts
In biopharma operations, consistency supports both quality and compliance. Variations in component performance can lead to process deviations or additional validation requirements.
NESPL provides:
- Precision-engineered spare parts
- Consistent product quality
- Components suitable for continuous production environments
- Equipment solutions aligned with process requirements
Reliable parts reduce unexpected downtime and support stable manufacturing cycles.
Compliance-Oriented Supply Support
Documentation and traceability are essential in regulated industries. Procurement and quality teams require clear specifications and reliable sourcing.
NESPL supports biopharma compliance needs by offering:
- Traceable supply chains
- Clear material and product documentation
- Components compatible with validation and audit processes
This simplifies internal approvals and strengthens readiness during inspections.
Minimizing Downtime in Critical Operations
Unplanned downtime in biopharma manufacturing can disrupt production schedules and increase operational costs.
By supplying dependable equipment and high-quality spare parts, NESPL helps facilities:
- Maintain consistent equipment performance.
- Reduce emergency replacements.
- Support preventive maintenance strategies.
Proactive sourcing of critical components improves operational resilience.
Supporting Precision in Process Control
Biopharma processes rely on precise control of pressure, flow, temperature, and sterility. The equipment and spare parts used must maintain that precision.
NESPL supplies components suitable for:
- Fluid handling systems.
- Controlled processing environments.
- Instrumentation and equipment interfaces.
This supports accurate process control and protects product integrity.
Responsive Service and Practical Coordination
Timely access to equipment and spare parts is essential in regulated manufacturing environments. Delays can affect production planning and maintenance schedules.
NESPL works with engineering, procurement, and maintenance teams to ensure:
- Availability of essential components.
- Practical coordination for urgent requirements.
- Reliable communication throughout the supply process.
This operational support helps facilities maintain continuity.
Conclusion
In biopharma manufacturing, the quality of equipment and spare parts directly influences process stability, compliance, and long-term performance.
NESPL supplies biopharma equipment’s spare parts specifically suited for regulated environments, supporting reliability, documentation readiness, and operational efficiency.
For organizations seeking a dependable partner with a clear understanding of the technical and regulatory demands of biopharma production, working with NESPL makes a measurable difference.